
Starting SUBLOCADE®
Getting Started on
SUBLOCADE
As you take your next step with SUBLOCADE, it's helpful to know what to expect
Whether you're new to recovery or starting your recovery journey again, SUBLOCADE is here to support you with a same-day start option that lasts all month long.

When you are ready for recovery, SUBLOCADE is ready for you
When you and your treatment provider decide to start SUBLOCADE, you may be able to begin that same day. What your first appointment looks like will depend on whether you're already taking buprenorphine.
- A same-day start option with stable medication delivery all month long so you can take your next step in recovery.*
- Already taking buprenorphine? Switch directly to your first SUBLOCADE injection the same day.
- Not already on buprenorphine? First, your treatment provider will give you a small test dose of oral buprenorphine and monitor you for an hour. If you respond well, you can receive your first SUBLOCADE injection the same day. After the first two injections, you will receive SUBLOCADE monthly.
*Buprenorphine should be started after the first sign of withdrawal, determined with your treatment provider.
†As a rapid induction protocol compared to standard of care initiation.

What happens after your first injection?
- Your second shot can be given as early as 1 week and up to 1 month after the first.
- This may help SUBLOCADE build up in your body faster.
How do I stay on track with monthly dosing?
- After your first two 300 mg injections of SUBLOCADE, you'll receive ongoing injections once a month (there will be at least 26 days between each injection).
- The recommended monthly dose of SUBLOCADE is 100 mg, but your treatment provider might adjust it to 300 mg based on how your body responds.
- No matter the dose, SUBLOCADE is designed to provide steady medication throughout the month—so you can stay focused on your recovery, not your medication.
Talk with your treatment provider about the dosing schedule that's right for you.

Your comfort matters
As with other long-acting injectables for opioid addiction, injection site pain was a commonly reported side effect with SUBLOCADE. Here are some of the findings from SUBLOCADE clinical trials:
- In one study, pain decreased over the first 5 minutes at each of 4 injection sites.
- In a study of the abdomen only, each monthly injection was less painful over time.
A closer look at getting started with once-monthly SUBLOCADE
Watch this video to learn what to expect with once-monthly SUBLOCADE.
Where on the body will you receive your SUBLOCADE injection?
SUBLOCADE offers 4 injection site options from the start:
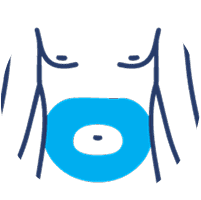
Abdomen
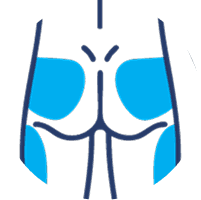
Buttock
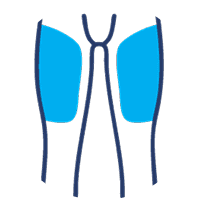
Thigh
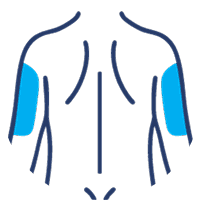
Back of the upper arm
Injection sites should be rotated each month.
If injections make you anxious, consider preparing for your appointments ahead of time.
You can even think of each monthly injection as your day for self-care or reflection. For example:
Be proud of yourself! Make a playlist just because you are proud of your progress.
Set reminders. Mark your appointments on your calendar so you can stay on track and know when to expect your treatment.
Spend time with loved ones. Bring someone with you. Sharing your experience fosters support and helps end stigma.
Reflect on yourself. Give yourself the time and space you need on your appointment day, and consider how a long walk, meditation, journaling, or another activity may help.

Have questions about cost and coverage?
Find out how 95% of people enrolled in the SUBLOCADE Copay Assistance Program‡ pay $0.
EXPLORE SAVINGS‡Eligibility restrictions apply. The Program benefit is valid for the out-of-pocket cost for SUBLOCADE only. It is not valid for any other out-of-pocket costs including costs associated with the administration of SUBLOCADE (for example, office visit or medication administration charges).
INDICATION
Prescription SUBLOCADE, with counseling and psychosocial support, is for adults with moderate to severe opioid addiction who have started treatment with a dose of oral buprenorphine or are being treated with buprenorphine.
IMPORTANT SAFETY INFORMATION
What is the most important information I should know about SUBLOCADE?
- Because of serious risk of potential harm or death from self-injecting SUBLOCADE into a vein (intravenously), it is only available through a restricted program called the SUBLOCADE REMS Program.
- SUBLOCADE is not available in retail pharmacies.
- Your SUBLOCADE injection will only be given to you by a certified healthcare provider.
- SUBLOCADE contains a medicine called buprenorphine which is an opioid that can cause serious and life‑threatening breathing problems, especially if you take or use certain other medicines or drugs.
- Talk to your healthcare provider about naloxone, which is a medicine that is available to patients for emergency treatment of an opioid overdose. If naloxone is given, call 911 or get emergency medical help right away to treat overdose or accidental use of an opioid.
- SUBLOCADE may cause serious and life-threatening breathing problems. Get emergency help right away if you: feel faint, dizzy, confused, sleepy or uncoordinated, have blurred vision or slurred speech, are breathing slower than normal or cannot think well or clearly.
- Do not take SUBLOCADE with certain medicines. Taking SUBLOCADE with other opioid medicines, benzodiazepines, alcohol, or other central nervous system depressants (including street drugs) can cause severe drowsiness, decreased awareness, breathing problems, coma, and death.
- In an emergency, have family members tell emergency department staff that you are physically dependent on an opioid and are being treated with SUBLOCADE.
- You may have detectable levels of SUBLOCADE in your body for several months after stopping treatment with SUBLOCADE.
Who should not receive SUBLOCADE?
Do not receive SUBLOCADE if you are allergic to buprenorphine or any ingredient in the prefilled syringe (delivery system: a biodegradable 50:50 poly(DL-lactide-co-glycolide) polymer and a biocompatible solvent, N-methyl-2-pyrrolidone (NMP)).
Before starting SUBLOCADE, tell your healthcare provider about all of your medical conditions, including if you have: trouble breathing or lung problems, a curve in your spine that affects your breathing, Addison's disease, an enlarged prostate, problems urinating, liver, kidney, gallbladder or mental health problems, alcoholism, head injury or brain problem, adrenal or thyroid gland problems.
Tell your healthcare provider if you are pregnant or breastfeeding or plan to become pregnant or breastfeed:
- If you receive SUBLOCADE while pregnant, your baby may have symptoms of opioid withdrawal at birth that could be life-threatening if not recognized and treated. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
- SUBLOCADE can pass into your breast milk and harm your baby. Talk to your healthcare provider about the best way to feed your baby during treatment with SUBLOCADE. Monitor your baby for increased drowsiness and breathing problems if you breastfeed during treatment with SUBLOCADE.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements before starting any new medicines and during or after stopping treatment with SUBLOCADE.
What should I avoid while being treated with SUBLOCADE?
- Do not drive, operate heavy machinery, or perform any other dangerous activities until you know how SUBLOCADE affects you. SUBLOCADE can make you sleepy, dizzy, or lightheaded, especially in the first few days after your injection and when your dose is changed.
- Do not drink alcohol or take prescription or over-the-counter medicines that contain alcohol during treatment with SUBLOCADE, because this can lead to loss of consciousness or even death.
What are the possible side effects of SUBLOCADE? SUBLOCADE can cause serious side effects, including:
- Trouble breathing. Taking SUBLOCADE with other opioid medicines, benzodiazepines, alcohol, or other central nervous system depressants can cause breathing problems that can lead to coma and death.
- Sleepiness, dizziness, and problems with coordination.
- Physical dependence or abuse.
- Liver problems. Call your healthcare provider right away if you notice any of these symptoms: your skin or the white part of your eyes turns yellow (jaundice), dark or "tea‐colored" urine, light colored stools (bowel movements), loss of appetite, pain, aching, or tenderness on the right side of your stomach area, or nausea.
Your healthcare provider should do blood tests to check your liver before you start and during treatment with SUBLOCADE.
- Allergic reaction. You may have rash, hives, itching, swelling of your face, wheezing, light‑headedness, feeling faint or loss of consciousness. Call your healthcare provider or get emergency help right away.
- Opioid withdrawal. Call your healthcare provider right away if you get any of these symptoms: shaking, sweating more than normal, feeling hot or cold more than normal, runny nose, watery eyes, goose bumps, diarrhea, vomiting, or muscle aches.
- Decrease in blood pressure. You may feel dizzy when you get up from sitting or lying down.
The most common side effects of SUBLOCADE include: constipation, headache, nausea, injection site itching, vomiting, increase in liver enzymes, tiredness, or injection site pain.
SUBLOCADE may affect fertility in males and females. Talk to your healthcare provider if this is a concern for you.
These are not all the possible side effects. Call your healthcare provider for medical advice about side effects.
To report a pregnancy or side effects associated with taking SUBLOCADE or any safety‑related information, product complaint, request for medical information, or product query contact PatientSafetyNA@indivior.com or 1‑877‑782‑6966. You are encouraged to report negative side effects of drugs to the FDA. Visit www.fda.gov/medwatch or call 1‑800‑FDA‑1088.
See full Prescribing Information, including Boxed Warning, and Medication Guide. For REMS information visit www.sublocadeREMS.com.